How Many Atoms Are Equal To 1.5 Moles Of Helium
1.5: Molecules and Moles
- Page ID
- 46529
And then far nosotros accept talked only most individual atoms or molecules, and about masses measured in atomic mass units. But individual molecules are difficult to manipulate in the laboratory, and chemists counterbalance their materials in grams, not in atomic mass units. To scale upward from the molecular level to the laboratory level, we utilise a unit chosen a mole. A mole of a substance is equal to as many molecules of that substance equally there are atoms of carbon-12 in exactly 12 g of carbon-12. This means that 1 mole of any substance is a weight, in grams, equal to that substance'due south molecular weight expressed in atomic mass units. Most important of all, by this definition, 1 mole of whatsoever substance contains the same number of molecules. The chemist can count atoms and molecules in the laboratory simply past weighing them. The discussion mole applies not just to molecules but also to atoms; in do, we speak of a mole of helium atoms likewise as of a mole of water molecules. The term gram-atom applied to a mole of atoms is no longer widely used.
Example 1.5.1
How many grams of each of the following substances are there in 1 mole of that substance: H2, H20 , CH3OH, octane (CeightH18), and neon gas (Ne)?
Solution
The molecular weights (in atomic mass units) of nigh of these substances accept been given in previous examples, and the atomic weight of neon is listed on the inside back cover. I mole of each substance is therefore:
-
-
H2 2.0160 thousand suspension C8Hxviii 114.23 g HtwoO xviii.0154 g intermission Ne twenty.179 g CH3OH 32.04 g break
-
Considering the weights listed in Case 9 give the correct relative weights of the molecules that are being weighed out, each of the quantities of material will comprise the aforementioned number of molecules. This is what makes the concept of moles useful. It is not fifty-fifty necessary to know what that number is, although we know it to exist 6.022 Ten 1023 ; it is called Avogadro's number and is given the symbol N Going from molecules to moles means a scale-upwards of 6.022 Ten 1023 times. Avogadro'due south number is also the conversion factor between atomic mass units and grams as units of mass: 1 g = 6.022 X 1023 amu. If we call back of the molecular weight as being the mass of a mole of substance, the units for molecular weight are grams per mole; if nosotros think of it every bit the actual weight of i molecule, the numerical value is unchanged but the units get atomic mass units per molecule. Both are correct.
Case i.five.2
One molecule of H2 reacts with ane molecule of Cltwo to form two molecules of hydrogen chloride gas, HCl. What weight of chlorine gas should be used in guild to react completely with one kilogram (kg) of hydrogen gas?
Solution
The molecular weights of H2 and Clii are 2.0160 g mole-ane and lxx.906 g mole-1, respectively. * Hence 1000 m of H2 contains:
-
-
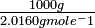 = 496.0 moles of H2 molecules
= 496.0 moles of H2 molecules
-
Without knowing how many molecules there are in a mole, nosotros tin still be sure that 496.0 moles of Cl2 will have the same number of molecules equally 496.0 moles or 1000 m of H2. How many grams of Clii are there in 496.0 moles? Since the molecular weight of Clii is seventy.906 g mole-1,
-
- 496.0 moles X lxx.906 g mole-1 = 35,170 g of Clii
1 kilogram equals 1000 one thousand, then 35,170 m is 35.17 kg. If 1.00 kg Htwo is made to react with 35.17 kg of C1two, the reaction will be complete and none of either starting textile will be left over.
* The expression "g mole-1" should be read as "grams per mole." In this note, a speed in miles per hour is written with units of "miles hr-I."
Example 1.v.3
How many molecules of \(H_2\) and \(Cl_2\) would be present in the experiment of Example ane.v.2?
Solution
In 496.0 moles of any substance, there will be
\[(496.0\; \cancel{mol}) \left(vi.022 \times 10^{23}\; \dfrac{molecules}{\cancel{mole}}\right) = 2.99 \times 10^{26}\; molecules\]
Equally a sobering case of simply how big Avogadro'southward number is, 1 mole of coconuts, each 14 centimeters (cm) in diameter, would fill a book as large every bit the unabridged planet world. The apply of moles in chemical calculations is the subject of the next affiliate, only the idea has been introduced hither because nosotros need to know how to scale upwardly from the molecular to the laboratory level.
Contributors and Attributions
-
R. Due east. Dickerson, H. B. Greyness, and Thou. P. Haight, Jr. Content was used from "Chemic Principles", an introductory college-level text for General Chemical science with permissionof the Caltech library and Harry B. Greyness, on behalf of the authors.
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_Chemical_Principles_(Dickerson)/01%3A_Atoms_Molecules_and_Ions/1.5%3A_Molecules_and_Moles

0 Response to "How Many Atoms Are Equal To 1.5 Moles Of Helium"
Post a Comment